Where medical devices are concerned, a UDI system is essential to qualitative healthcare. If UDI becomes part of recall management, it can greatly increase both patient safety and operational efficiency. This article examines the many uses of UDI in medical device recall management.
What is the Unique Device Identifier (UDI)?
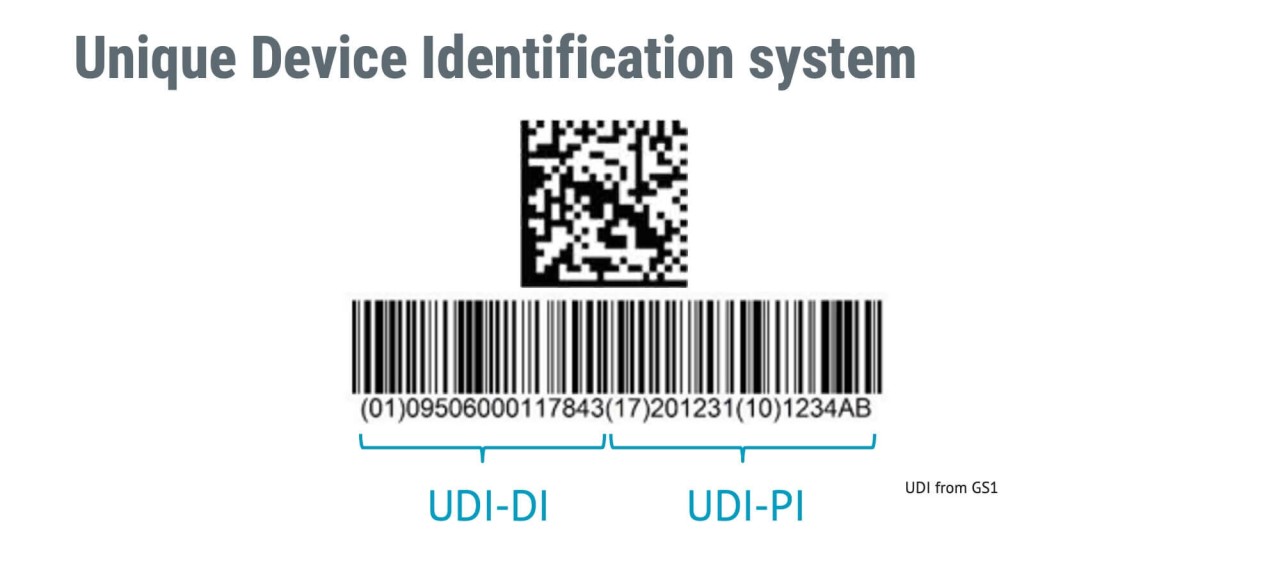
Within the supply and market chain, the Unique Device Identifier (UDI) is a code that is placed to medical devices in order to identify and trace them. Typically, the code is generated by the maker of the medical equipment; however, it is also possible for an economic operator to develop the code so long as they get authorization from the manufacturer.
In 2007, a request was made to the Food and Drug Administration (FDA) to adopt standards that would limit the number of errors that had an impact on patients and would make it possible to identify and recall defective devices much more quickly. A national tracking system was conceived of by the Food and Drug Administration (FDA), which then initiated trial programs to build the system. A total of more than thirty million dollars was saved in supply contracting as a result of the pilot programmes’ implementation of universal product codes as mandatory components of reimbursement claims in health insurance situations. Additionally, the data demonstrated that defective devices were found significantly more quickly than in the past, which increased supply chain efficiency.
Benefits of UDI for Medical Devices
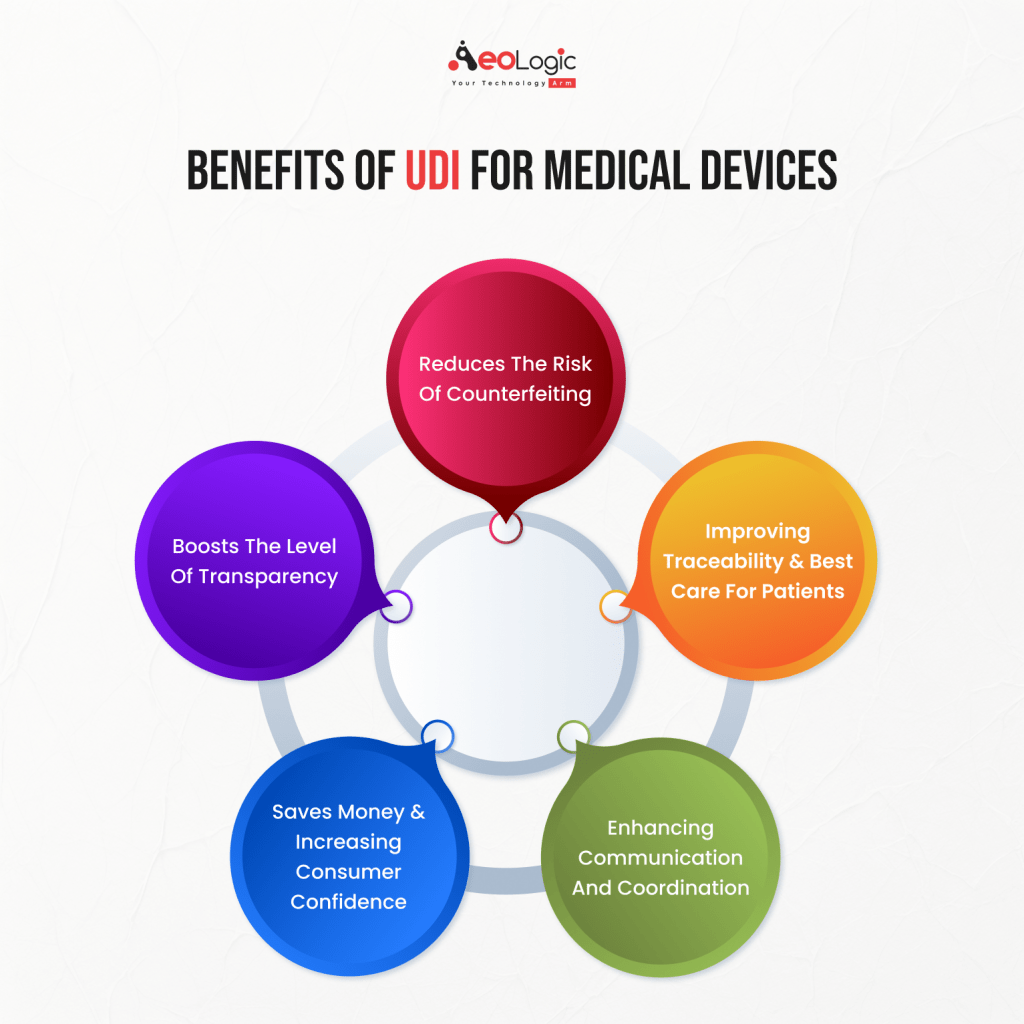
UDI for medical devices makes the MedTech business more uniform so that patients, manufacturers, and providers all have to follow the same rules. It makes sure that everyone involved is getting the same benefits.
Reduces the Risk of Counterfeiting
Forbes says that between $1.7 trillion and $4.5 trillion worth of fake goods are sold every year, but they can’t say for sure. Fraudsters go after all kinds of businesses and use cheap labour to make cheap goods.
Adding UDIs stops fake medical devices from being sold in the medical device business. Because GS1, the Health Industry Business Communications Council (HIBCC), and the International Council for Commonality in Blood Banking Automation (ICCBBA) choose the numbers, the addition of UDIs brings to light fake devices. Because of this, the number can’t be copied or put into the GUDID very easily.
Also read: Cybersecurity in Healthcare: Importance and Use Cases
Improving Traceability
Recall management must emphasize traceability. UDIs track such a transparent trail from manufacturing to end-use. With this traceability, all those involved will be able to monitor the life of a device, making it easier to manage recalls.
Enhancing Communication and Coordination
Communicating effectively is the key focus of recalls. UDIs help to create a more coordinated relationship between manufacturers, health care providers and relevant regulatory bodies. UDI’s major advantage over other forms of recall management is that everyone is in-the-know, with the bottom line being improved communication.
Saves money
Healthcare workers did not have to keep track of inventory data before they had to enter it into the GUDID system. Even if they did, the database could have been a forgotten administrative job.
UDI for Medical devices makes sure that all service locations are always updating their stock. As a result, there is a better system for keeping track of inventory and making sure that the right inventory is bought. Additionally, UDIs encourage quick recalls, so it costs less to take back a faulty product or go through the claims process.
Boosts the level of transparency
UDI for Medical devices makes everything more clear, from makers to patients. It saves data that lets you see through the curtain between a provider and a manufacturer. Both sides are sure that the device is in the right supply chain, has been updated, and is being used properly. The UDIs saved in the database also show possible trends in broken devices, which gives a clear picture of how devices are made and used.
UDIs also make things clearer for patients. When you make a UDI, you also make the public UDI database, AccessGUDID. This database can be used by anyone to make sure a gadget is legal.
Do you know? Role RFID Traceability in Medical Device Industry
The Best Care for Patients
UDIs are good for manufacturers, healthcare professionals, and institutions, but patients are the ones who gain the most. Anti-counterfeit protects patients from devices that give them wrong information, break quickly, or put their lives in danger. Patients can be sure that the devices being used on them have been through a thorough process of standardisation.
Also, the identification makes sure that the provider always has the right gadget and knows how to fully use it. As soon as there is a problem with a recalled or broken product, doctors can get to the bottom of it with little trouble for patients. Patients don’t have to wait a long time for a device to be put back on.
Increasing Consumer Confidence
This is why consumer confidence in medical devices is so important. UDI’s recall management transparency and efficiency help build public confidence in medical devices. If a highly efficient recall system is in place then patients and other health care professionals need not worry.
Also Read: Future Healthcare Technology That Will Change the World
Final Words
The advantages of UDI for medical devices are abundant and are of profound importance for patient safety, operational efficiency, and public confidence. The implementation and use of UDIs are a major step forward in improving the quality and safety of healthcare.
To learn more about how UDI can change healthcare, reach out to Aeologic Technologies. We’re ready to help you bring this smart tech into your medical work.